Coagulants for water purification in the pool: how to choose + application rules
Owners of suburban areas with pools are well aware of the problem of gradual pollution of water and the walls of the structure. Despite its cyclical movement and regular passage through filters, a cloudy precipitate and a health hazardous suspension are almost inevitable.
There are effective remedies to combat the loss of water quality that are worth familiarizing yourself with. Do you agree?
Using coagulants to purify water in the pool, you can quickly and productively get rid of these problems and threats. We suggest that you familiarize yourself with information that illuminates the principle of action of substances, their varieties and the specifics of choosing the optimal composition.
The article describes in detail the process of using coagulants, gives recommendations on keeping the private pond clean, and gives folk methods to combat its clouding. The information provided is supported by photo collections and videos.
The content of the article:
The principle of coagulants
These substances have the property of combining microscopic particles of various pollutants, debris, heavy metals and biological particles into a bulk jelly-like mass with the subsequent transition of this emulsion into flakes.
In this form, a suspension that could seep through pool filters, is delayed by the mesh and ceases to circulate in the water body of the pool.
Contamination must be removed from the bottom and surface. The top film can be removed with an ordinary net.
When using automated tank care products, the sediment will linger on the filter, from which it can be easily removed by regular washing. To do this, you can use a stream of water under pressure.
In addition to cleaning water in the pool, coagulants are actively used for sewage treatment.
Varieties and selection rules
All products that are commercially available contain organic or inorganic binders active substances.
They are based on sulfates, polyoxysulfates or chlorides of the following metals:
- Aluminum (aluminum) Al.
- Iron (ferrum) Fe.
- Titanium (titanium) Ti.
- Magnesium (magnesium) Mg.
Manufacturers indicate the composition in Latin, therefore, along with the name of the active substance, its Latin name will be duplicated. The composition of the drug can be found either on the back of its packaging, or in the instructions.
Organic coagulating agents
The most popular coagulants are produced on the basis of aluminum polyoxychloride (polyaluminium chloride), its second name is hydroxychloride. Aluminum polyoxychloride has several advantages over inorganic reagents.
Among them are the key:
- High quality cleaning, which means that less reagent is required compared to sulfates.
- Low residual aluminum content in water after the coagulation process, which reduces the frequency of water changes in the pool.
- Flakes are faster, which means that the settling period is reduced.
- Reduced residual salt content, which allows less frequent replacement of water in the pool.
- Effect duration - a high degree of purification is maintained even at a reduced level of ambient temperature.
When hit in the ground, this reagent does not lead to a disruption of the natural balance in the region. The process of dissolution in water is simplified, since the tool does not require prolonged mixing.

When using this tool, you do not need to apply additional protective equipment. It is enough to use gloves to protect the skin of the hands from irritation. A respirator is also recommended.
Inorganic formulations with coagulating effect
The most popular inorganic compounds for coagulation are:
- Aluminum sulphate (aluminum sulfate).
- Iron sulfate (ferrous sulphate).
- Titanium dioxide (titanium dioxide).
Aluminum sulphate has ease of use, as it is diluted in water without prolonged sedimentation.Its disadvantage is its sensitivity to the predominance of acid or alkaline components in water.
The values of the tester should not go beyond the range of a neutral pH level of 6.5 - 7.5, otherwise the effectiveness of the coagulant decreases sharply. The reagent is also sensitive to low temperatures and its use in the spring-autumn period is excluded.

The disadvantage of its use is the increased (compared with hydrochloride) release of salts, which changes the pH level in water. This makes it necessary to level it, and also increases the frequency of water changes in the pool, since in addition to salt, when used in water, the aluminum dose is exceeded.
Iron sulfate allows you to get rid of the unpleasant odor of hydrogen sulfide, neutralize oily contaminants and get rid of the high content of heavy metals. According to these properties, the reagent is superior to aluminum-containing reagents.
It does not completely dissolve in water, a small part of the precipitate remains, not exceeding 1% by weight of the reagent.

Titanium dioxide has the highest percentage of cleaning. This reagent has a pronounced bactericidal effect, and can be used without additional chlorination.
Titanium dioxide has several advantages over derivatives of iron and aluminum. It reduces the settling time. According to this indicator, the reagent has no analogues.

Titanium dioxide is produced in Russia and abroad, so it can be found and purchased from suppliers.
The disadvantage of the reagent is its high cost. After applying titanium dioxide, the water becomes suitable for drinking, which is unnecessary for the pool. It is worth making a choice in favor of budget analogues, based on aluminum.
When choosing a cleaning product, you need to pay attention to the integrity of the package. Some reagents are sensitive to oxygen and are actively oxidized when interacting with it. This applies to iron-based coagulants.
The reagents in liquid form contain a ready-made solution, which simplifies the process of use, but the price is higher. It is more profitable to purchase the product in the form of a powder.
It is enough for more applications, moreover, it costs less. This also applies to cartridges that are installed in the filter pump.
Comparison of coagulants with improvised means
In the absence of filters or their weak power, the problem of flowering water in the pool appears. The lack of necessary reagents forces the use of improvised substances.
Most popular remedies:
- hydrogen peroxide;
- potassium permanganate;
- diamond green on alcohol.
They have a disinfecting effect. The effect of their use lasts temporarily and leads to consequences that need to be considered separately.

After applying the peroxide on the surface of the water, flakes of dirty foam appear. They are removed mechanically. Even after two days, the process of oxygen evolution will continue, which gives uncomfortable tactile sensations. If water with dissolved peroxide comes into contact with the skin, slight tingling will begin.
Do not allow swallowing of this aqueous solution, as well as ingestion into the respiratory system. This causes irritation of the mucous membranes. Peroxide allows water to cool more slowly, as it increases its density. However, peroxide cannot replace full purification with a coagulant.
Potassium permanganate diluted in water has a disinfecting property until its color changes from pale pink to light brown or green.

The composition of zelenok includes alcohol and triphenylmethane dye. There is no exact data on how this coloring pigment affects a person when ingested. With prolonged contact of the water in which the brilliant green is dissolved, with the walls of the pool, the material changes color.

These reagents cannot serve as a complete replacement for coagulants, since they do not bind a fine suspension. They can only disinfect water for a short time, while hazardous heavy metals and substances invisible to the eye do not disappear. They continue to be in the tank.
Step-by-step instructions for use
Before cleaning with preparations, it is necessary to measure the acid-base balance. It must be done with a special device (pH tester) or litmus papers.
The optimal level is from 7.5 to 8.0. If the result is less than the specified range, then you need to add alkali, if more, then add acid.

After bringing the pH balance to neutral, you can begin to disinfect. This is a necessary measure to prevent infection by infectious pathogens. For this purpose, use chlorine-containing tablets, which, unlike liquid agents, do not emit an antiseptic dosed during dissolution.
# 1: Calculation of proportions depending on the displacement
Before pouring the reagent, you need to calculate the displacement. To determine it, it is necessary to calculate the volume of the pool. For the calculation, it is necessary to measure the length, width and depth of the tank. If the pool has a round shape, then you need to measure the diameter and depth.
All measurements must be done in meters.
- formula for calculating the volume of a rectangular container: length * width * depth;
- formula for calculating the volume of a round container: depth * 6.28 * square radius.
The values obtained will be the displacement in liters. The dose of the substance is calculated based on this value. If the degree of water pollution can be visually determined as strong, an increased dose may be necessary, in this case it is necessary to fill 1.3 of the recommended by the manufacturer and can reach 25 ml per 1 m3.
Coagulant consumption can be reduced if flocculants are used as an adjunct. These substances are used specifically for the formation of flakes and for weighting to facilitate filtering processes. They are added within two minutes after entering the coagulant.
# 2: Preparation and pouring of the solution
Manufacturers offer to purchase a reagent in three states:
- powder;
- liquid;
- briquetted.
Liquid reagent needs preliminary preparation. It must be diluted in water in a ratio of 1: 5. Next, you need to disable the filtering system. If this is not done, she will quickly clog dirty flakes. The prepared solution must be poured into a watering can, and evenly poured over the entire surface.
The use of a dispenser is suitable for weekly care, since the concentration of the substance in the water for cleaning heavy contaminants will be too low.
Coagulant briquettes are placed in special cartridges. They are installed in filtration pumps. When water passes through the briquette, it carries with it part of the reagent back to the pool.

If the reagent has a powdery consistency, you must first prepare a concentrated solution. Mass fraction of aluminum sulfate should be 15%. To do this, the contents of the bag must be dissolved in equal to its weight amount of water. A dilution ratio of 1: 1.
To prepare a solution with a mass fraction of aluminum sulfate or polyoxychloride of less than 15%, you need to use the formulas:
- K1 = K * D / D1 - to calculate the mass of the reagent in kilograms;
- V = K - K1 = K * (1 - D / D1) - to calculate the amount of water in liters.
Explanation of the formulas:
- K1 - mass of reagent;
- K - the required mass of the solution;
- D - mass fraction (%) of aluminum sulfate (polyoxychloride) in solution;
- D1 - mass fraction (%) of aluminum sulfate (polyoxychloride) in the starting reagent.
The resulting solution must be poured into the pool. You can make a solution in advance, it is well stored unchanged for a year.
# 3: Cleaning the surface and bottom after coagulation
After 10-12 hours after the start of coagulation, you need to clean the bottom and surface of the precipitate. Used for this purpose water vacuum cleaner. This device has two hoses, one of which is attached to the tube.

The surface of the water in the temporary structure built for the summer season is cleaned either with a vacuum cleaner or mounted water intakes made in the form of skimmers. This is a special bowl that connects to the filter system and collects debris from the surface.
In stationary pools, this is a mechanism built into the system that is used to collect water from the surface for subsequent cleaning.

In the skimmer bowl there is a grid that prevents the passage of large debris that can damage the filter system. As the bowl is filled, it must be cleaned manually. Such a system is able to completely clear the surface within an hour of active work.
A practical but costly solution to the problem of cleaning the pool is to buy a robot vacuum cleaner. An overview of popular models is given in this article.
Tips for keeping your pool clean
In order to prevent the pool from clogging, it is necessary to regularly do chlorination with special tablets. After chlorination, once every 2 weeks cleaning is carried out with reagents. After the reagents, the pool is cleaned with a water vacuum cleaner. After that, the filtration system is turned off and the filter is washed.
After 12 hours, the filtration system is turned on and a cloudy suspension is removed from the bottom and surface of the pool. During this period, clean the filter again. It must be cleaned by moving the hoses according to the model of the pump and turning on the backwash. Part of the old water from the pool is removed, and quartz sand is washed in the filter.
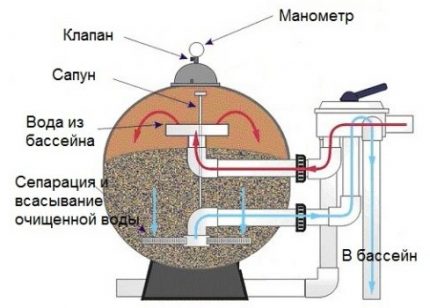
After flushing, the hoses must be returned to their original working condition and, if necessary, pour fresh water into the pool. Since with the collapse of the coagulant in the water, an excess amount of the products of the action of the reagent accumulates over time, every two months a complete replacement of water in the swimming pool is necessary.
The cleanliness of the pool is largely dependent on the effectiveness of the filter plant and the quality of the water. Some craftsmen, in an effort to save money, do do-it-yourself filter.
Conclusions and useful video on the topic
Water purification by coagulation by the example of experience:
Cleaning cloudy sediment after coagulation:
Good reagents should be bought from reliable manufacturers, be sure to pay attention to the mass fraction of the active substance. The higher the percentage of its content, the more efficient and economical its use will be.
If the desired result was not achieved when using the drug, then the dose should be increased. If this does not help, it is better to drain the water from the pool, as an unknown composition may be harmful.
Have experience using coagulants to clean the pool, or maybe you know effective methods to eliminate pollution? Or have questions about the topic? Please share your opinion and leave comments.


 How to choose a filter for the pool: types of units and the rules for the right choice
How to choose a filter for the pool: types of units and the rules for the right choice  Pool heat pump: selection criteria and installation rules
Pool heat pump: selection criteria and installation rules  How to choose a pump for the pool: a comparative overview of different types of units
How to choose a pump for the pool: a comparative overview of different types of units  Dehumidifiers for pools: how to choose and calculate the optimal dehumidifier
Dehumidifiers for pools: how to choose and calculate the optimal dehumidifier  Pumps for fountains and waterfalls: how to choose and install yourself
Pumps for fountains and waterfalls: how to choose and install yourself  How to make a pool with your own hands: step-by-step instructions for the construction
How to make a pool with your own hands: step-by-step instructions for the construction  How much does it cost to connect gas to a private house: the price of organizing gas supply
How much does it cost to connect gas to a private house: the price of organizing gas supply  The best washing machines with dryer: model rating and customer tips
The best washing machines with dryer: model rating and customer tips  What is the color temperature of light and the nuances of choosing the temperature of the lamps to suit your needs
What is the color temperature of light and the nuances of choosing the temperature of the lamps to suit your needs  Replacement of a geyser in an apartment: replacement paperwork + basic norms and requirements
Replacement of a geyser in an apartment: replacement paperwork + basic norms and requirements
It’s really difficult to choose. I would like to know how safe water is for the health of swimmers, after cleaning with coagulants. I am especially worried about the health of children. So, if a lot of aluminum accumulates in the body, Alzheimer's disease can develop. Is titanium harmless? I prefer potassium permanganate or peroxide, it may not be as effective, but safe.
Titanium does not absorb and does not accumulate in the human body, but is eliminated from it after several hours. And think about it - well, here, in the USA, coagulant producers would have been condemned for a long time if it were dangerous to health.
I would gladly use only greenback and potassium permanganate, but they do not clean completely, and they can also paint the tiles. We have to use hydrochloride - it is both more effective and less dangerous for the skin and mucous membrane than sulfates. I follow safety precautions, I carefully clean the bottom after reagents: so far, none of the households have been poisoned with reagents and no one has allergies either.
We bought an inflatable pool for grandchildren. Children are thrilled, flop there almost all day.But a problem arose - although the size of the pool is not the most gigantic, it is not possible to change the water in it every day. What is better to clean it in such a situation? Will there be enough improvised tools listed in the material or will I have to buy all these sulfates and dioxides?
Hello. There are many products that can help you clean your water. For example, one of this is ordinary salt, calculated as 5 kg per 1 ton of water, green water - 3 bottles per 10 cubic meters, 700 ml of hydrogen peroxide per 1 cubic meter (bathe only a day after purification), again, the above in article means. But, of course, it would be best, after all, to change the water daily and to clean the surface.